SUPERA KVALITETA provides business consulting services for activities related to:
computer system validation, small application validation, cleaning validation, process validation, equipment qualification
 Validation of information, laboratory and process systems and maintenance of computer systems in a validated state in compliance with applicable guidelines and guides: EU GMP Annex 11, ISPE GAMP 5, FDA 21 CFR Part 11 (for systems using electronic records and signatures) covers activities:
Validation of information, laboratory and process systems and maintenance of computer systems in a validated state in compliance with applicable guidelines and guides: EU GMP Annex 11, ISPE GAMP 5, FDA 21 CFR Part 11 (for systems using electronic records and signatures) covers activities:
- assistance in the preparation and / or review of validation documentation for all phases of the computer system life cycle (planning, specifications, development, installation qualification, operation qualification, performance qualification, "go-live")
- assistance in producing the necessary procedures (SOP - Standard Operating Procedure)
- risk analysis
- audit of vendor
- performing education
 We provide support for the creation and validation of small applications (Access, Excel, FileMaker) used in the GxP environment that must be validated.
We provide support for the creation and validation of small applications (Access, Excel, FileMaker) used in the GxP environment that must be validated.
 We also provide support services for compliance of systems, computer systems and procedures with data integrity requirements in accordance with applicable guidelines.
We also provide support services for compliance of systems, computer systems and procedures with data integrity requirements in accordance with applicable guidelines.
 Cleaning validation - the purpose of cleaning validation is to confirm that the cleaning process is effective and to ensure that the medicinal product is safe by preventing cross-contamination.
Cleaning validation - the purpose of cleaning validation is to confirm that the cleaning process is effective and to ensure that the medicinal product is safe by preventing cross-contamination.
Ceaning validation and maintenance of validated status in compliance with applicable Guidelines and Guides: EU GMP Vol 4. Part I and Part II, EU GMP Annex 15, PIC / S PI 006, APIC Guidance on aspects of cleaning validation in active pharmaceutical ingredient plants, FDA Guide to inspections validation of cleaning processes, ICH Guidelines, VICH Guidelines, covers activities:
- assistance in preparation and / or review of validation documentation (validation master plan (VMP), cleaning validation protocol, cleaning validation report, descriptions of cleaning procedures - SOPs, work instructions, etc.)
- pomoć u izradi matrice opreme proizvoda i grupiranju proizvoda
- assistance in preparation of product equipment matrix and product grouping
- assistance to set eligibility criteria and to calculate limits for the rest of the product
- risk analysis
- performing education
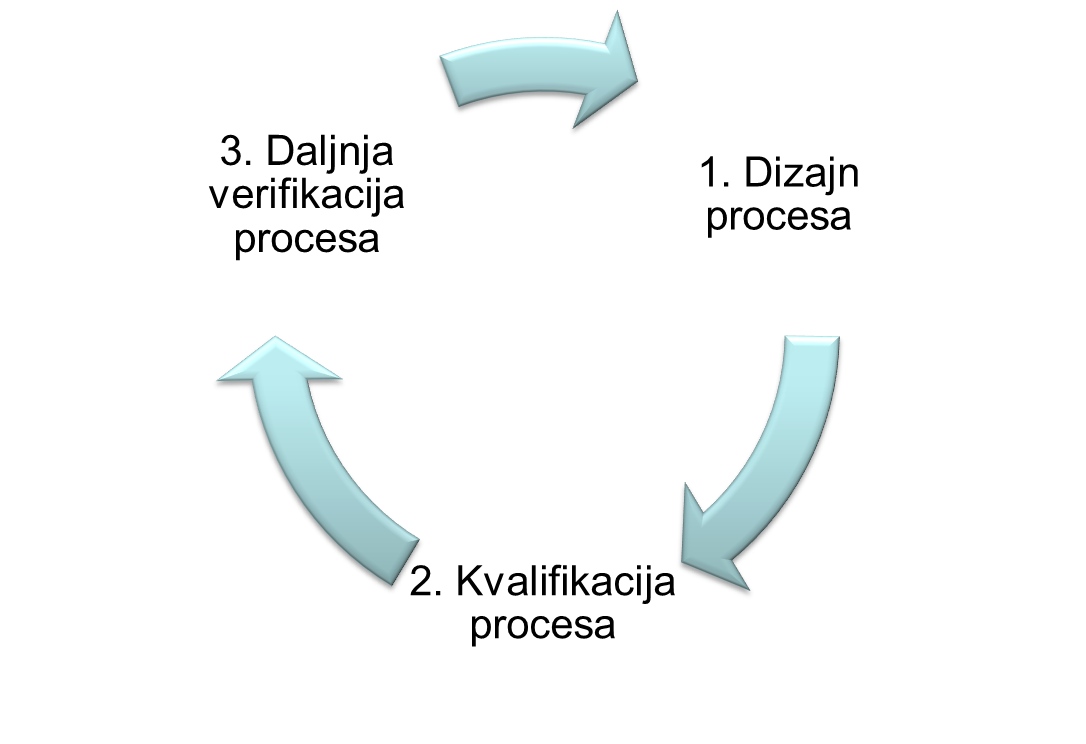
Process validation - documented evidence that a process run within set parameters can effectively and reproducibly produce a product that complies with predefined specifications and quality characteristics.
Process validation in accordance with applicable guidelines and guides: EU GMP Annex 15, EU GMP Chapter 5, EMA Guideline on Process Validation, FDA Guidance for Industry Process Validation, includes activities:
- assistance in the preparation and / or review of validation documentation (validation master plan (VMP), validation protocol (validation plan), validation report)
- risk analysis
- consulting in the field of modern approaches to process development and validation: Quality by Design (QbD), Process Analytical Technology (PAT), Continued Process Verification
- performing education
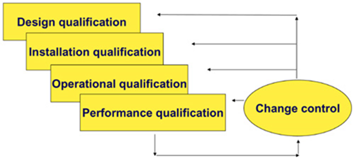 Equipment qualification - process that proves and documents that the equipment is functioning properly and that it really produces the expected results.
Equipment qualification - process that proves and documents that the equipment is functioning properly and that it really produces the expected results.
Equipment qualification in accordance with applicable guidelines and guides: EU GMP Vol 4. Part I and Part II, EU GMP Annex 15, EMA Guideline on Process Validation, PIC / S PI 006, WHO Annex 4 - Appendix 6, FDA Guidance for Industry Process Validation, includes activities:
- assistance in the preparation and / or review of qualification documentation (User Requirements Specification (URS), Design Qualification - DQ), Factory Acceptance Testing (FAT), Site Acceptance Testing - SAT), Installation Qualification - IQ, Operational Qualification - OQ, Performance Qualification - PQ
- risk analysis
- performing education
We organize and perform in-house trainings in English language for computer system validation, small application validation, cleaning validation, process validation, equipment qualification and other similar topics.
