Equipment qualification in accordance with applicable guidelines and guides: EU GMP Vol 4. Part I and Part II, EU GMP Annex 15, EMA Guideline on Process Validation, PIC / S PI 006, WHO Annex 4 – Appendix 6, FDA Guidance for Industry Process Validation, includes activities:
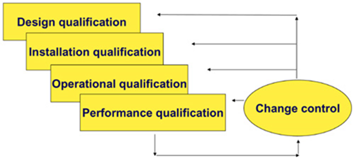
- assistance in the preparation and / or review of qualification documentation (User Requirements Specification (URS), Design Qualification – DQ), Factory Acceptance Testing (FAT), Site Acceptance Testing – SAT), Installation Qualification – IQ, Operational Qualification – OQ, Performance Qualification – PQ
- analizu rizika
- performing education
We organize and perform in-house trainings in English language for computer system validation, small application validation, cleaning validation, process validation, equipment qualification and other similar topics.